Introduction
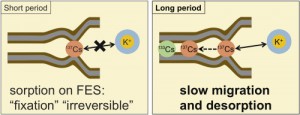
Vast areas of north-eastern regions of Japan has been contaminated by radionuclides released from the accident of the TEPCO’s Fukushima Dai-ichi nuclear power plants. In particular, radioactive cesium, namely 137Cs, cause many problems due to its relatively long half-life (30.1 years), including decontamination and the management of contaminated soil wastes. To settle such problems, we need to know long-term behaviors of radioactive Cs in soils. Cesium is reported to be strongly fixed micaceous to so-called frayed edge sites (FES) in minerals in soils by interlayer collapse. Nevertheless,the long-term desorption behaviors is of great importance, which is not yet fully understood in terms of the mechanisms, partly because of its slowness and also of the existence of re-sorpton. In this research, we performed the long-term desorption experiments of 137Cs and 133Cs from the real contaminated soils in the presence of varying concentrations of competing cations. The resorption of desorbed Cs was diminished by adding cation-exchange resin as an adsorbent.
Experimental
The contaminated soils collected in Fukushima prefecture after 40 days since the accident were used. Soils were suspended in dilute KCl solution together with a dialysis bag containing cation exchange resins (Dowex 50w-X8,K+ form).The dialysis bags were exchange 10 times in 3 months with new ones. 133Cs and 137Cs released from the soils and captured by the resigns were quantified by ICP-MS and Ge detector.
Results & discussion
From all samples, more sup>137Cs than that in short-term ion exchange experiments with high concentration of competing ions (ex. K+ and NH4+) was desorbed after 3 months. In the initial period, the desorption of 137Cs was suppressed by high concentration of K+; the effect was diminished in time, and the desorption at the high concentration of K+ exceeded that at the low concentration. Desorption of 133Cs was different from that of 137Cs, which suggested that these isotopes exist in different chemical forms, reflecting the differences in their residence time in soil.
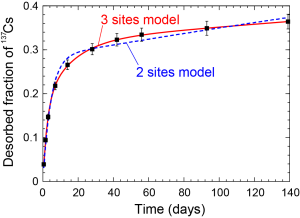
A desorption model with 3 sites were fit to the observed desorption curves. The half-lives of the desorption from the slowest site fell into the range of years, indicating that a part of 137Cs is strongly fixed soils and is desorbed slowly.
Murota, K., Saito, T.*, Tanaka, S., “Desorption kinetics of cesium from Fukushima soils”, J. Environ. Radioact. 153, 134-140 (2016).
Introduction
Vast areas of north-eastern regions of Japan has been contaminated by radionuclides released from the accident of the TEPCO’s Fukushima Dai-ichi nuclear power plants. In particular, radioactive cesium, namely 137Cs, cause many problems due to its relatively long half-life (30.1 years), including decontamination and the management of contaminated soil wastes. To settle such problems, we need to know long-term behaviors of radioactive Cs in soils. Cesium is reported to be strongly fixed micaceous to so-called frayed edge sites (FES) in minerals in soils by interlayer collapse. Nevertheless,the long-term desorption behaviors is of great importance, which is not yet fully understood in terms of the mechanisms, partly because of its slowness and also of the existence of re-sorpton. In this research, we performed the long-term desorption experiments of 137Cs and 133Cs from the real contaminated soils in the presence of varying concentrations of competing cations. The resorption of desorbed Cs was diminished by adding cation-exchange resin as an adsorbent.

Experimental
The contaminated soils collected in Fukushima prefecture after 40 days since the accident were used. Soils were suspended in dilute KCl solution together with a dialysis bag containing cation exchange resins (Dowex 50w-X8,K+ form).The dialysis bags were exchange 10 times in 3 months with new ones. 133Cs and 137Cs released from the soils and captured by the resigns were quantified by ICP-MS and Ge detector.
Results & discussion
From all samples, more sup>137Cs than that in short-term ion exchange experiments with high concentration of competing ions (ex. K+ and NH4+) was desorbed after 3 months. In the initial period, the desorption of 137Cs was suppressed by high concentration of K+; the effect was diminished in time, and the desorption at the high concentration of K+ exceeded that at the low concentration. Desorption of 133Cs was different from that of 137Cs, which suggested that these isotopes exist in different chemical forms, reflecting the differences in their residence time in soil.
A desorption model with 3 sites were fit to the observed desorption curves. The half-lives of the desorption from the slowest site fell into the range of years, indicating that a part of 137Cs is strongly fixed soils and is desorbed slowly.

Murota, K., Saito, T.*, Tanaka, S., “Desorption kinetics of cesium from Fukushima soils”, J. Environ. Radioact. 153, 134-140 (2016).
