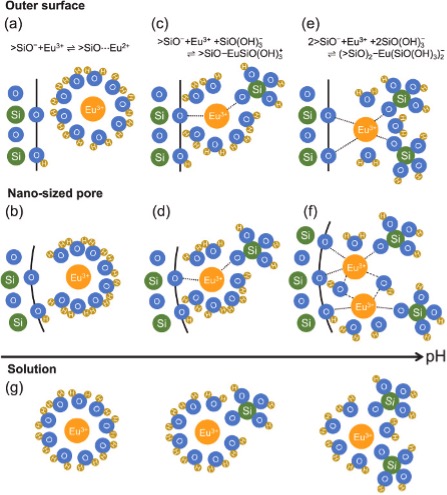
We analyzed the adsorption states of europium aqua ions (Eu3+) on mesoporous silicas with different pore distributions in comparison with nonporous silica using time-resolved laser-induced fluorescence spectroscopy. The parallel factor analysis was used to differentiate the contribution of different chemical species of Eu3+ to the fluorescence signal and determine the influence of the pore size on each chemical species. The results show that Eu3+ mainly form outer-sphere complexes with silica below pH 6, where Eu3+ adsorption is low. Within nano-sized pores, distortion of the hydration structure and a decrease in the hydration number were suggested in this pH range. As the concentration of the silicate ions derived from the dissolution of silica increases with increasing pH, Eu3+ form the silica/Eu3+/silicate ternary surface complexes. Within nano-sized pores, the concentration of silicate ions decreases due to the overlap of the electric double layer, which inhibits the formation of the ternary surface complex. Furthermore, at high pH, Eu3+ multinuclear complexes formed only on the mesoporous silica surface. This adsorption behavior specific to nano-sized pores could not be concluded by macroscopic adsorption experiments alone because the amount of Eu3+ adsorbed per unit surface area did not differ between the mesoporous and nonporous silicas. Consideration of the silicate complexes should be indispensable in future studies on the adsorption of lanthanide ions using mesoporous silica.
Murota, K., Aoyagi, N., Mei, H., Saito, T., “Hydration states of europium(III) adsorbed on silicas with nano-sized pores”, Appl. Geochem. 152, 105620 (2023).