Background
Vast areas of the eastern part of Japan have been contaminated by a short-lived radioisotope of cesium (Cs), 137Cs, whose half-life is 30.1 years, from the accident of the Fukushima Dai-ichi Nuclear Power Plant in 2011. Most of contaminated soils in residential areas have been stripped and gathered for future disposal. On the other hand, air dose rates in forests are still high. Understanding the behaviors of Cs+ in soils is thus crucial for the safety assessments of the disposal of contaminated soil in future and the evaluation of long-term exposure of residents living near forest areas. It is known that Cs+ is strongly sorbed to micaceous minerals. However, the desorption of Cs+ at a trace sorption level with time in the presence of different salt ions is not well understood. In this study, we conducted long-term sorption and desorption experiments of Cs+ with illite and vermiculite, which are micaceous minerals, at room temperature to study the effects of sorption time and co-existing cations on the desorption.
Method
A small amount of Cs+ (50 nM Cs+ spiked with 900 Bq 137Cs) was sorbed to the illite and vermiculite in the presence of 1 mM K+ or Ca2+, or 1 mM K+ and 100 mM Ca2+ over 8 weeks, which was then desorbed in the presence of Prussian blue (PB) nanoparticles, which is a strong Cs sorbent, over 12 weeks. The PB nanoparticles were used to inhibit the re-sorption of desorbed Cs+.
Results and discussions
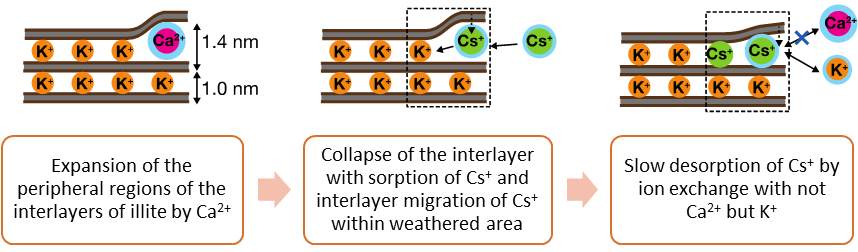
More than 90% of Cs+ was sorbed to the minerals in the presence of Ca2+; meanwhile, only 50 to 70% of Cs+ was in the presence of K+. For all samples other than the illite with Ca2+ (Ca-illite), more than 80% of Cs+ were desorbed within a few days, and almost all Cs+ was desorbed at the end of the experiment. The large and fast desorption of Cs+ indicated a large part of Cs+ sorbed to these minerals were indeed labile in the presence of a strong sorbent like PB nanoparticles. These desorption trends were hardly influenced by a change of the sorption time. The desorption of Cs+ from the Ca-illite was slow, taking more than one month before 80% desorption for the sample with 1-day sorption, and the desorption amount only reached less than 90%. This slow desorption of Cs+ from the Ca-illite became even slower with the sorption time from one day to two weeks, and only 70% of sorbed Cs+ was desorbed at the end of the experiment for the latter. The mechanisms of Cs+ desorption from the Ca-illite was quantitatively explained by fitting to a pseudo first-order desorption model, suggesting that 30 – 40% of Cs+ was sorbed to the peripheral region of the interlayer of the Ca-illite and diffused into the interior part. The rest of sorbed Cs+ can be desorbed relatively fast. As this Cs+ was most likely sorbed to frayed edge sites in the Ca-illite, these results suggested that a part of the sorbed Cs+ (70 – 60%) was labile. Thus, the expansion and collapse of the peripheral regions of the interlayers induced by co-existing cations and interlayer migration of Cs+ are important processes constraining the sorption and desorption of Cs+ to/from the micaceous minerals. In addition, compared with the desorption from the pure minerals examined in this study, the desorption of Cs+ from real soils was slower likely due to weathering and/or the formation of aggregates.
Murota, K.*, Tanoi, K., Ochiai, A., Utsunomiya, T., Saito, T., “Desorption mechanisms of cesium from illite and vermiculite”, Appl. Geochem., in press (2020).
This research was partly supported by ‘Grant-in-Aid for Scientific Research (B)’ (Grant No. 15H04246) from the Japan Society for the Promotion of Science.